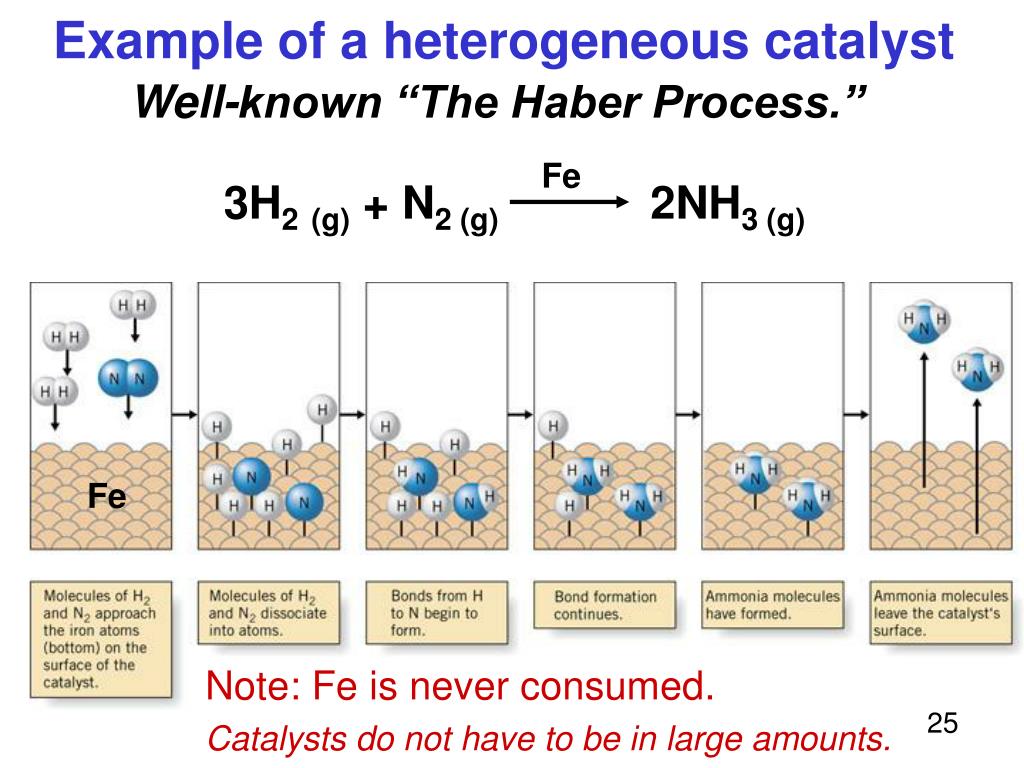
Heterogeneous Catalyst Chemistry Examples . Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. One or more bonds break concomitantly with adsorption. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. examples of heterogeneous catalysis. most examples of heterogeneous catalysis go through the same stages: Homogeneous catalysis and heterogeneous catalysis. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. an example is alkene binding by platinum. The simplest example of this is the reaction between ethene and hydrogen in the. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. In this case, the barrier to dissociation. Hydrogenation of ethylene on a heterogeneous catalyst. When a molecule of hydrogen adsorbs to the catalyst surface,. reactions that are facilitated by catalysts can be divided into two major classes:
from www.slideserve.com
for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. examples of heterogeneous catalysis. Homogeneous catalysis and heterogeneous catalysis. The simplest example of this is the reaction between ethene and hydrogen in the. One or more bonds break concomitantly with adsorption. an example is alkene binding by platinum. reactions that are facilitated by catalysts can be divided into two major classes: Hydrogenation of ethylene on a heterogeneous catalyst. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for.
PPT Part IV Activation Energy Jespersen Chapter 14 Sec 5
Heterogeneous Catalyst Chemistry Examples most examples of heterogeneous catalysis go through the same stages: One or more bonds break concomitantly with adsorption. Homogeneous catalysis and heterogeneous catalysis. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. reactions that are facilitated by catalysts can be divided into two major classes: an example is alkene binding by platinum. examples of heterogeneous catalysis. In this case, the barrier to dissociation. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. The simplest example of this is the reaction between ethene and hydrogen in the. Hydrogenation of ethylene on a heterogeneous catalyst. most examples of heterogeneous catalysis go through the same stages: When a molecule of hydrogen adsorbs to the catalyst surface,. Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of Heterogeneous Catalyst Chemistry Examples Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. When a molecule of hydrogen adsorbs to the catalyst surface,. The simplest example of this is the reaction between ethene and hydrogen in the. In this case, the barrier to dissociation. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. One or. Heterogeneous Catalyst Chemistry Examples.
From www.chemistrystudent.com
Heterogeneous Catalysis (ALevel) ChemistryStudent Heterogeneous Catalyst Chemistry Examples In this case, the barrier to dissociation. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. reactions that are facilitated by catalysts can be divided into two major classes: Homogeneous catalysis and heterogeneous catalysis. examples of heterogeneous catalysis. most examples of heterogeneous. Heterogeneous Catalyst Chemistry Examples.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube Heterogeneous Catalyst Chemistry Examples Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. most examples of heterogeneous catalysis go through the same stages: Homogeneous catalysis and heterogeneous catalysis. an example is alkene binding by platinum. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. The simplest example of this is the reaction between. Heterogeneous Catalyst Chemistry Examples.
From brainly.in
Difference between homogenous and heterogenous catalysis with example Heterogeneous Catalyst Chemistry Examples most examples of heterogeneous catalysis go through the same stages: an example is alkene binding by platinum. In this case, the barrier to dissociation. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. examples of heterogeneous catalysis. When a molecule of hydrogen adsorbs to the catalyst. Heterogeneous Catalyst Chemistry Examples.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Heterogeneous Catalyst Chemistry Examples examples of heterogeneous catalysis. Homogeneous catalysis and heterogeneous catalysis. One or more bonds break concomitantly with adsorption. an example is alkene binding by platinum. In this case, the barrier to dissociation. Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. most examples of heterogeneous catalysis go through the same stages: One or more of the reactants are. Heterogeneous Catalyst Chemistry Examples.
From www.mdpi.com
Catalysts Free FullText Switchable StimuliResponsive Heterogeneous Catalyst Chemistry Examples Hydrogenation of ethylene on a heterogeneous catalyst. Homogeneous catalysis and heterogeneous catalysis. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. examples of heterogeneous catalysis. an example is alkene binding by platinum. heterogeneous catalysts take the form of either metals or ionic. Heterogeneous Catalyst Chemistry Examples.
From alchetron.com
Heterogeneous catalysis Alchetron, the free social encyclopedia Heterogeneous Catalyst Chemistry Examples In this case, the barrier to dissociation. The simplest example of this is the reaction between ethene and hydrogen in the. reactions that are facilitated by catalysts can be divided into two major classes: Hydrogenation of ethylene on a heterogeneous catalyst. Homogeneous catalysis and heterogeneous catalysis. One or more of the reactants are adsorbed on to the surface of. Heterogeneous Catalyst Chemistry Examples.
From www.mdpi.com
Catalysts Free FullText General and Prospective Views on Oxidation Heterogeneous Catalyst Chemistry Examples for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. In this case, the barrier to dissociation. examples of heterogeneous catalysis. The simplest example of. Heterogeneous Catalyst Chemistry Examples.
From www.slideshare.net
Catalysis Heterogeneous Catalyst Chemistry Examples One or more bonds break concomitantly with adsorption. The simplest example of this is the reaction between ethene and hydrogen in the. Hydrogenation of ethylene on a heterogeneous catalyst. an example is alkene binding by platinum. most examples of heterogeneous catalysis go through the same stages: Homogeneous catalysis and heterogeneous catalysis. for example, oxides of iron placed. Heterogeneous Catalyst Chemistry Examples.
From www.youtube.com
Difference Between Homogeneous Catalysis and Heterogeneous Catalysis Heterogeneous Catalyst Chemistry Examples In this case, the barrier to dissociation. When a molecule of hydrogen adsorbs to the catalyst surface,. Homogeneous catalysis and heterogeneous catalysis. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. examples of heterogeneous catalysis. an example is alkene binding by platinum. One or more bonds break. Heterogeneous Catalyst Chemistry Examples.
From exovalyuk.blob.core.windows.net
Catalytic Group Definition at William Barham blog Heterogeneous Catalyst Chemistry Examples The simplest example of this is the reaction between ethene and hydrogen in the. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. Homogeneous catalysis and heterogeneous catalysis. an example is alkene binding by platinum. Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. In this case, the. Heterogeneous Catalyst Chemistry Examples.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog Heterogeneous Catalyst Chemistry Examples One or more bonds break concomitantly with adsorption. Hydrogenation of ethylene on a heterogeneous catalyst. most examples of heterogeneous catalysis go through the same stages: examples of heterogeneous catalysis. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. When a molecule of hydrogen adsorbs to the catalyst surface,. Revision. Heterogeneous Catalyst Chemistry Examples.
From www.researchgate.net
(PDF) Types of catalysis, heterogeneous and homogenous catalysis Heterogeneous Catalyst Chemistry Examples Homogeneous catalysis and heterogeneous catalysis. The simplest example of this is the reaction between ethene and hydrogen in the. most examples of heterogeneous catalysis go through the same stages: One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. Hydrogenation of ethylene on a heterogeneous catalyst. In this case, the barrier. Heterogeneous Catalyst Chemistry Examples.
From www.researchgate.net
Use of heterogeneous catalysis in various chemistry sectors Download Heterogeneous Catalyst Chemistry Examples One or more bonds break concomitantly with adsorption. In this case, the barrier to dissociation. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. Hydrogenation. Heterogeneous Catalyst Chemistry Examples.
From www.cambridgescholars.com
From Solid State Chemistry to Heterogeneous Catalysis Cambridge Heterogeneous Catalyst Chemistry Examples When a molecule of hydrogen adsorbs to the catalyst surface,. Hydrogenation of ethylene on a heterogeneous catalyst. The simplest example of this is the reaction between ethene and hydrogen in the. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. One or more bonds break. Heterogeneous Catalyst Chemistry Examples.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Transition metals Heterogeneous catalysis Heterogeneous Catalyst Chemistry Examples When a molecule of hydrogen adsorbs to the catalyst surface,. reactions that are facilitated by catalysts can be divided into two major classes: Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. Hydrogenation of ethylene on a heterogeneous catalyst. Homogeneous catalysis and heterogeneous catalysis. One or more of the reactants are adsorbed on to the surface of the catalyst. Heterogeneous Catalyst Chemistry Examples.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram Heterogeneous Catalyst Chemistry Examples Revision notes on 1.8.4 homogeneous & heterogeneous catalysts for. heterogeneous catalysts take the form of either metals or ionic compounds, which can either be bare or serve as. an example is alkene binding by platinum. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as. Heterogeneous Catalyst Chemistry Examples.
From celimoko.blob.core.windows.net
Catalyst Reactions Examples at Jenny McNear blog Heterogeneous Catalyst Chemistry Examples When a molecule of hydrogen adsorbs to the catalyst surface,. One or more of the reactants are adsorbed on to the surface of the catalyst at active sites. for example, oxides of iron placed on alumina (a chemical compound with the formula al 2 o 3) are widely used as heterogeneous. examples of heterogeneous catalysis. Homogeneous catalysis and. Heterogeneous Catalyst Chemistry Examples.